Drug-Patent Linkage System
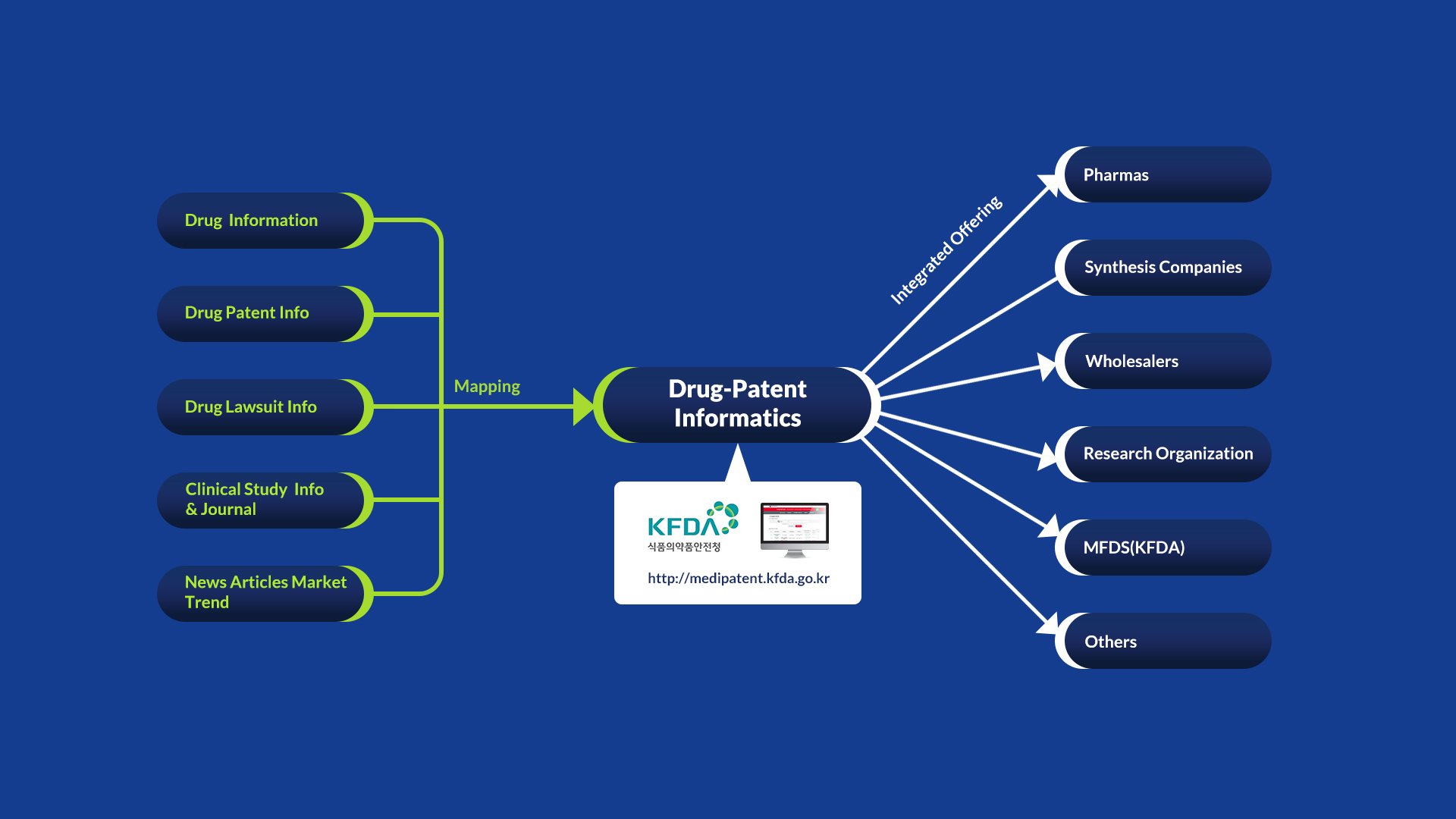
Drug-Patent Informatics
EXIA(prior to Klasia) had made and managed Drug-Patent Informatics website that currently operated by Ministry of Food and Drug Safety(http://medipatent.mfds.go.kr/)
Planning
1) Item Selection
Analysis of all trialed and untrialed market exclusivity items
The following summarizes the strategic approach of the patent. approval linkage. Klasia selects competitive items after analyzing all items in the Green List from MFDS(similar to the Orange Book)

2) Item Selection
Classification items
After selection of items, we have classified into the untrialed and market exclusivity one.
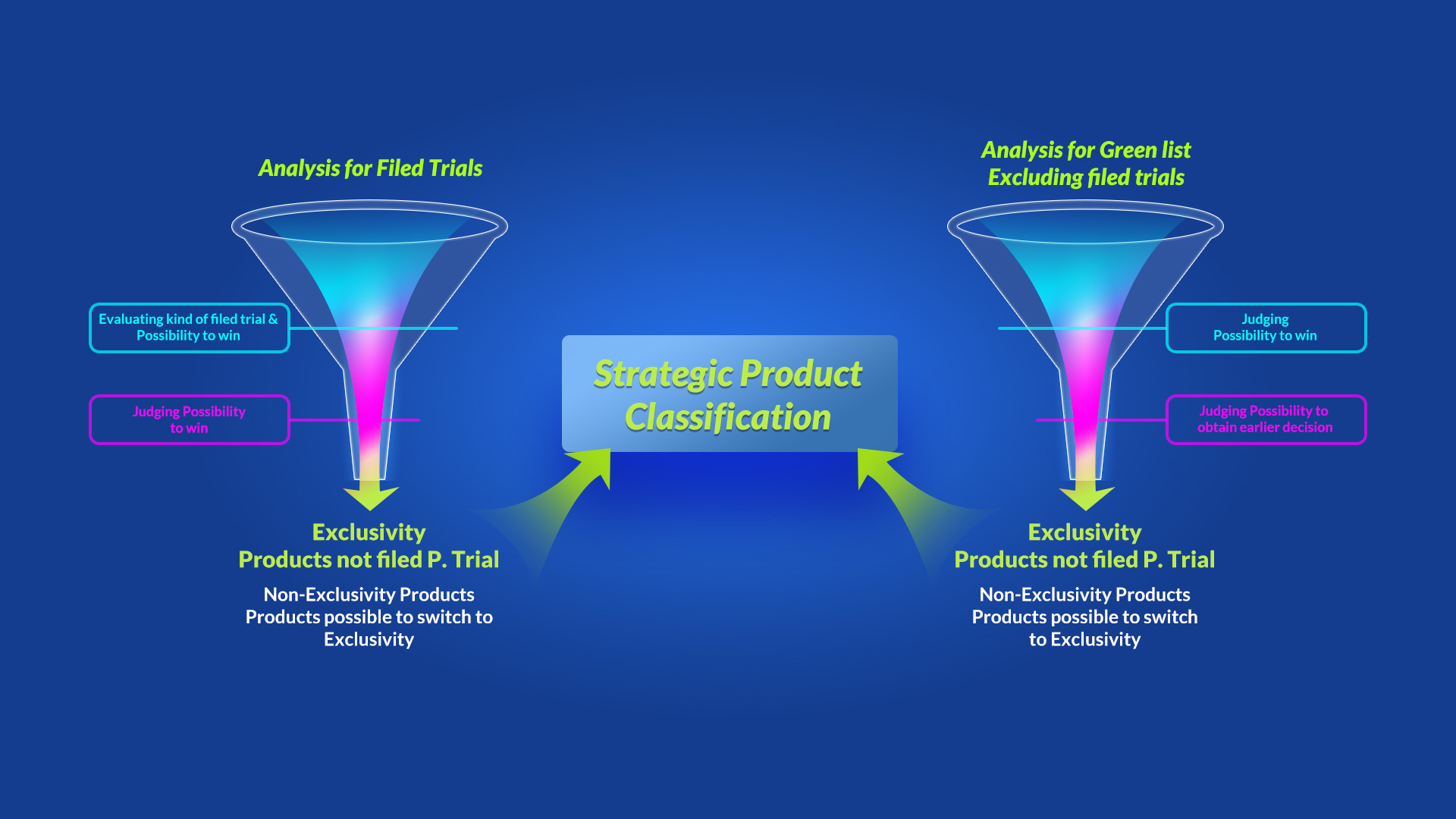
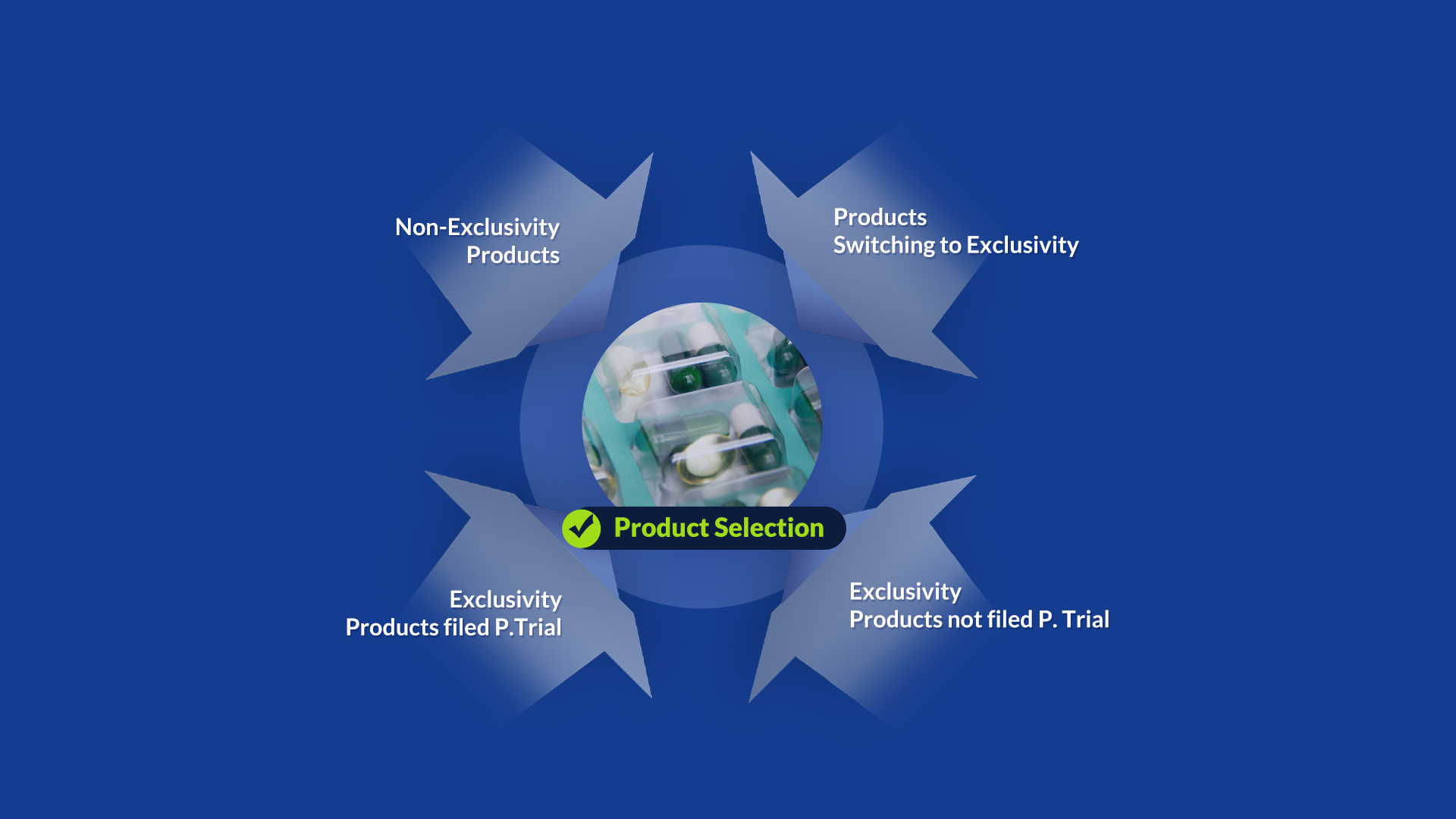
Finally, we classified the items into four categories and identified differentiated business items through strategic analysis.
3) Making strategic partners
After confirming business items, we make a contract with domestic or foreign API supplier that having tecnology and experience to make non-infringing API.
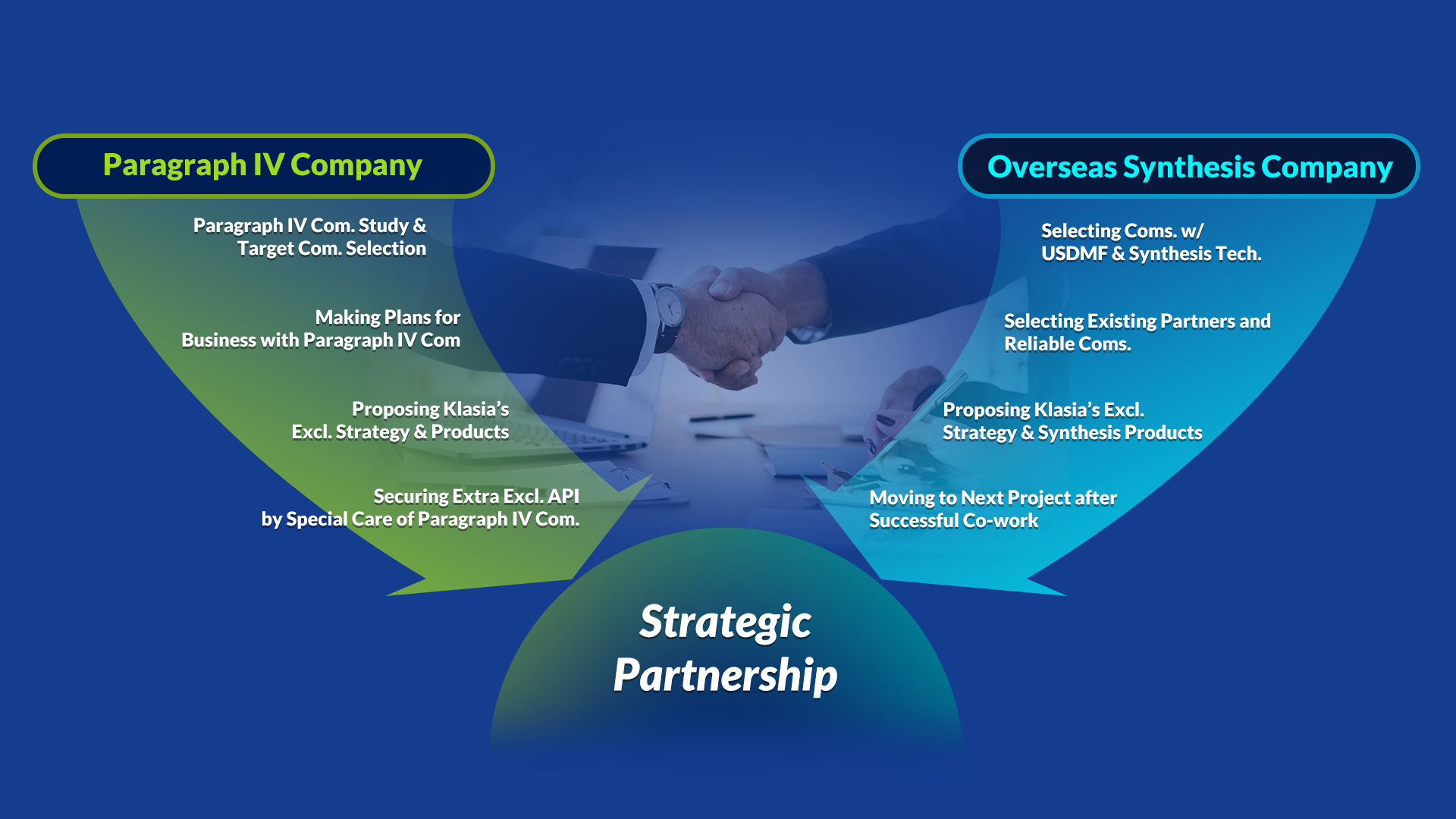
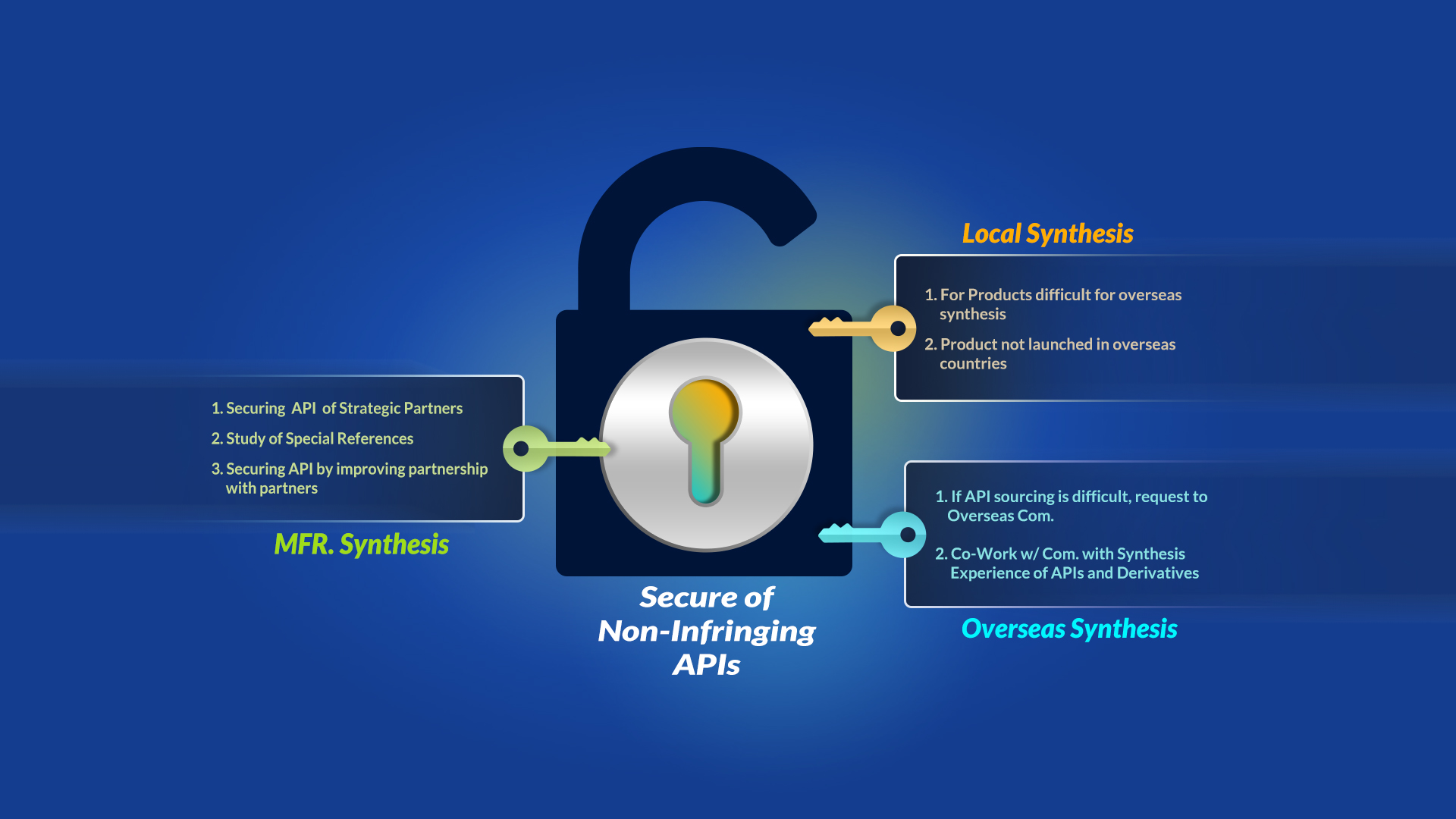
We are ready to support your competitiveness with our strategic partners and market exclusivity process.
4) How to Collaborate
Klasia has the differentiated items and can maximize your profit with the synergy of R&D and distribution.
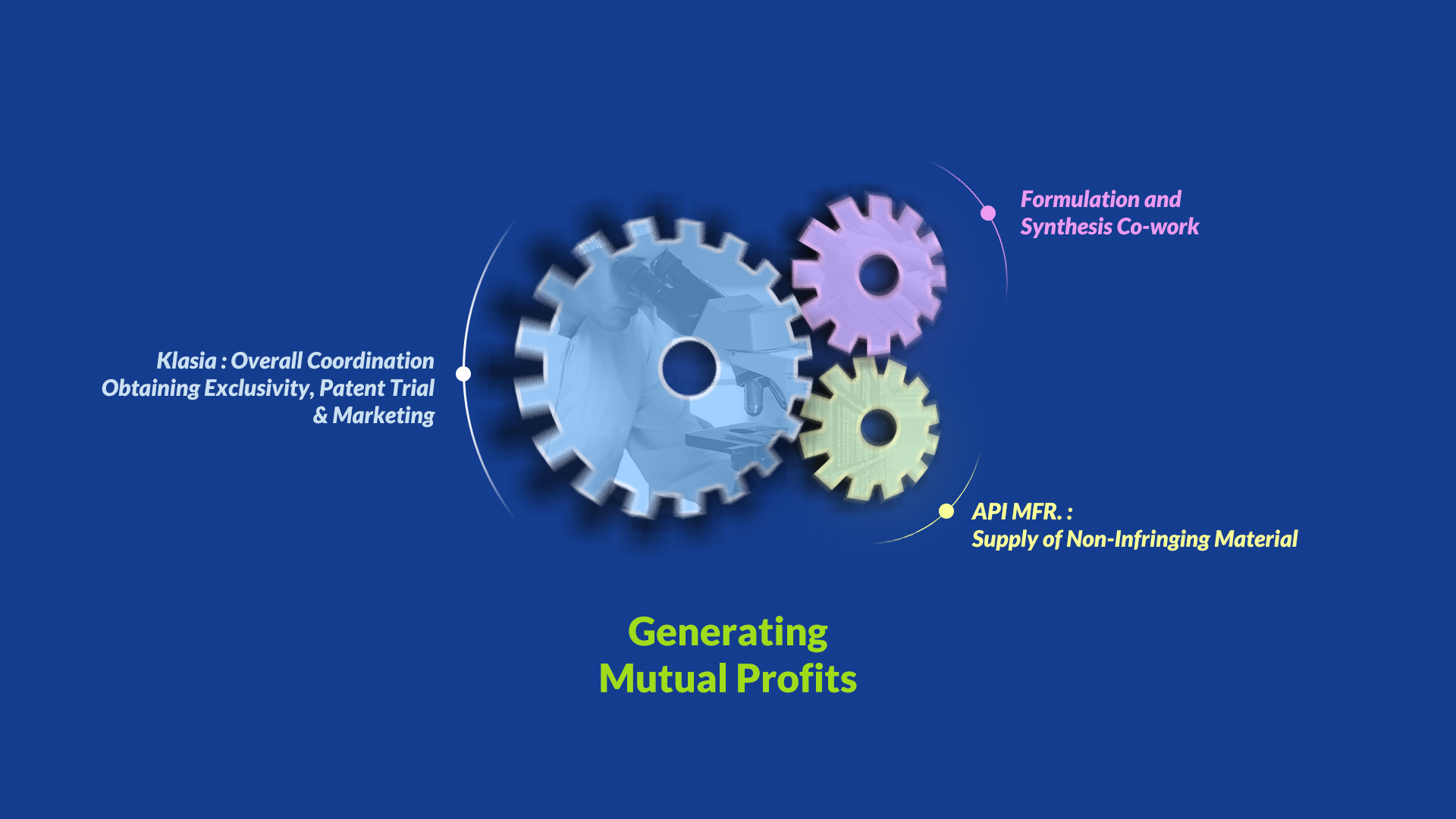
In addition to differentiated items and securement of ingredients, klasia supports your R&D by providing patent evasion and invalidation.
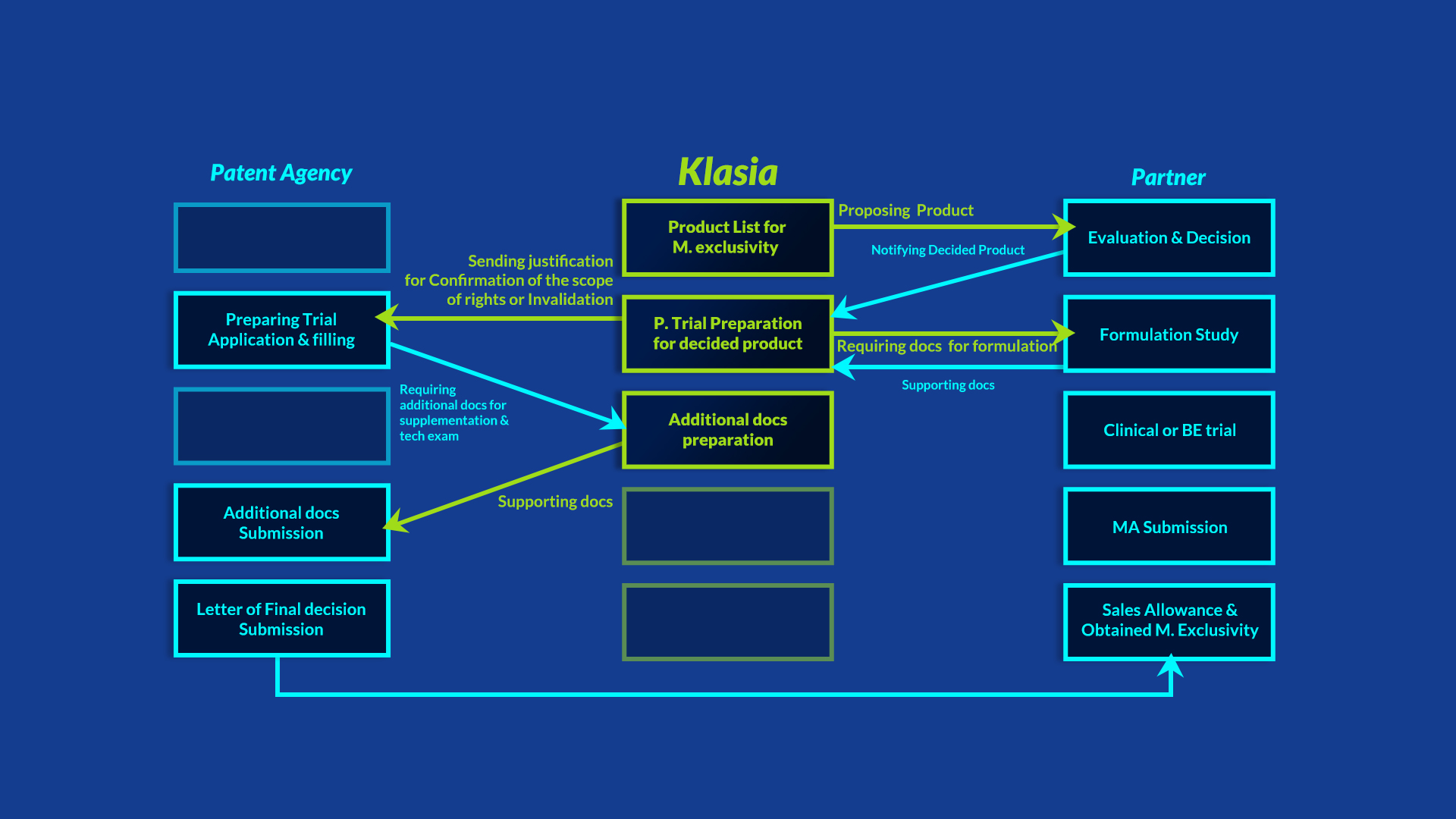
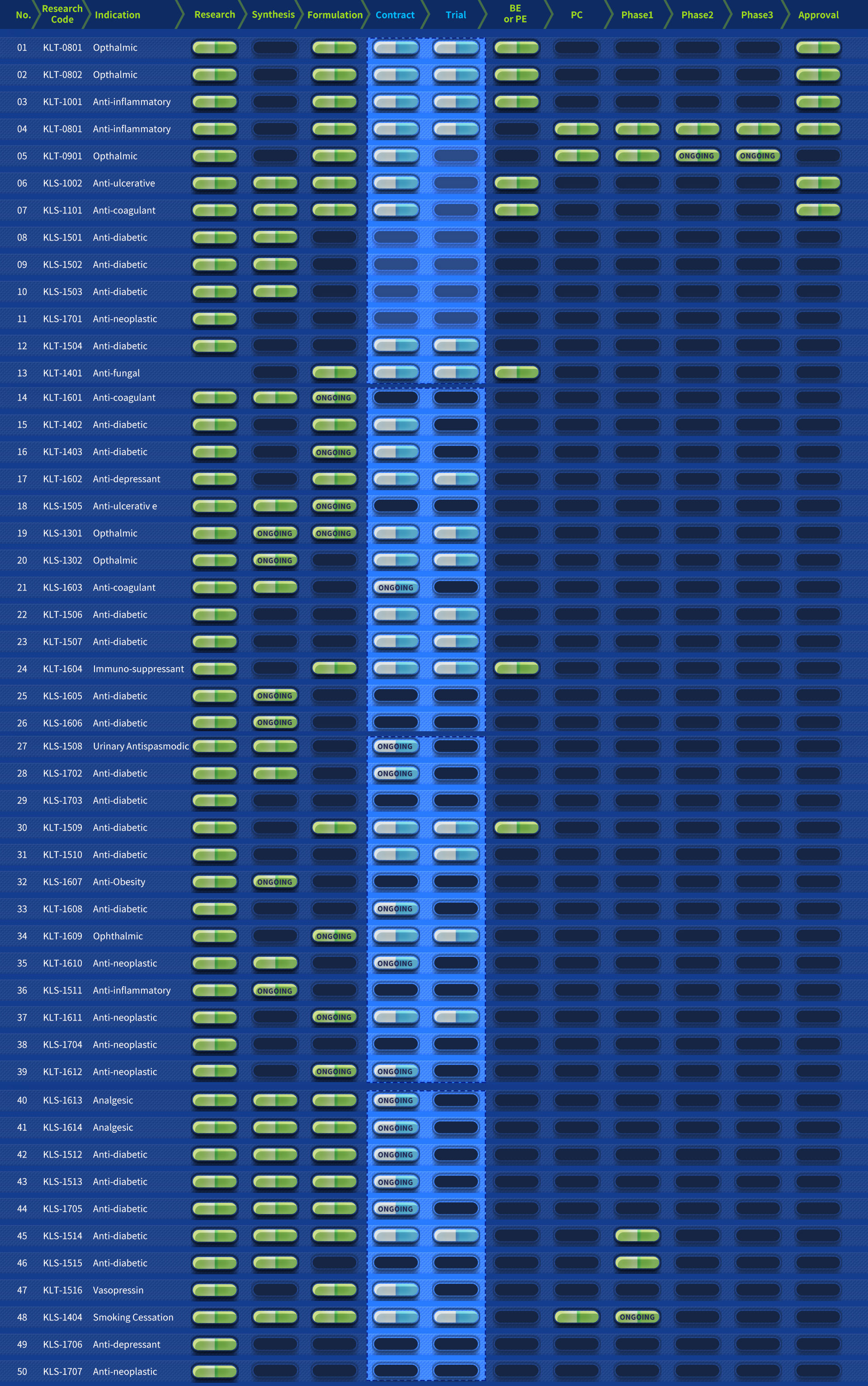
R&D Pipelines